
News
In 2020, it was rated as a national specialized and new "little giant" enterprise by the Ministry of industry and information technology
2023-06-25 305
1. Brief introduction and application of antioxidants
Antioxidants are a class of substances that inhibit or retard undesired oxidation reactions. In broad sense, most substances with a reducing function can act as antioxidants. Antioxidants have different requirements and regulations depending on their specific use and function in different fields. Generally weak reducing agents with stable chemical and physical properties and good biosafety performance can be used as antioxidants. In the field of food and pharmaceuticals, typical natural antioxidants, such as vitamin E or ascorbic acid, can be added to food and beverage formulations as antioxidants to inhibit oxidative deterioration during preservation and prolong storage time In the field of plastics, rubber and other polymer materials, the addition of antioxidants can alleviate and inhibit the thermal degradation of polymer materials, inhibit aging reactions, maintain the good performance of the material and extend the service life of the material. Antioxidants are a class of chemicals that, when present in small amounts in a polymer system, delay or inhibit the oxidation process of the polymer, thereby preventing the polymer from aging and extending its service life, also known as “antiage”.

The production process of plastic products basically needs to add antioxidants, including polyolefin industry antioxidants accounted for about 50% of the antioxidant plastics market, PVC, styrene resins and other industries antioxidants accounted for about 20% of the antioxidant plastics market, the remaining amount added in engineering plastics, polyurethane, thermosetting resins, etc. The global trend of antioxidants will continue to be mainly hindered phenols about 50%, supplemented by phosphite esters about 40%, but based on improving the binary compounding system is moving toward a more comprehensive ternary compounding system, such as hindered phenols / phosphite / benzofuranone, hindered phenols / phosphite / phosphate and benzofuranone and other types. As a result of increased global environmental awareness, "green" antioxidant products based on vitamin E are also becoming available. In short, efficient and harmless compounding multifunctional antioxidants will become the leading direction of development in the 21st century.

2. Mechanism of antioxidant action
The essence of the thermal oxidation process is a series of free radical chain chemical reactions in which the molecular chemical bonds in organic compounds are broken and decomposed under the action of heat, light or oxygen to recreate reactive radicals such as -OH, O- and ROO- or to produce hydroperoxides. A series of free radical chain reactions can occur between radicals to produce new substances or new structures, resulting in fundamental changes in the structure and properties of the original organic compound. In order to inhibit undesired reactions, substances that can scavenge free radicals or provide functional groups such as H- are needed, mainly by adding antioxidants. The main function is to eliminate the newly generated free radicals or to promote the decomposition of hydroperoxides and prevent the chain reaction from taking place. Antioxidants that can eliminate free radicals include compounds such as aromatic amines and hindered phenols and their derivatives, which are called primary antioxidants; antioxidants that can decompose hydroperoxides include organic compounds containing phosphorus and sulfur, which are called auxiliary antioxidants.
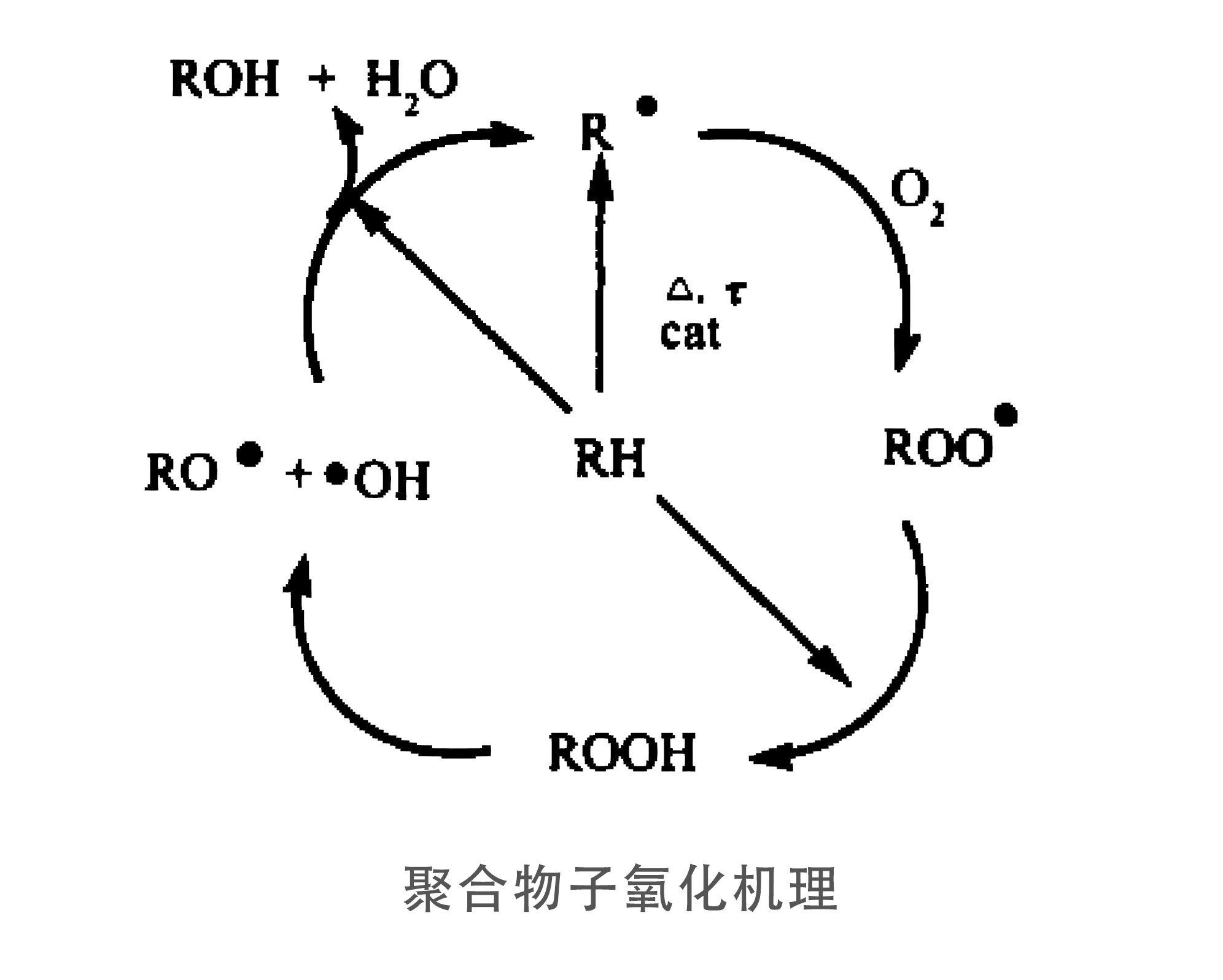
聚合物子氧化机理:Oxidation mechanism of polymer
3. Types and applications of antioxidants
In order to improve the antioxidant capacity of polymers, antioxidants are added to scavenge the generated active radicals or decompose peroxides. There are different ways to classify antioxidants according to their functional and structural characteristics.
3.1 Phenolic antioxidants
Phenolic antioxidants are widely used in plastics, synthetic fibers, rubber, food, and other industries. Yongjun Feng et al. developed a high-performance antioxidant for polypropylene (PP) using a low-molecular-weight hindered phenolic antioxidant (AO) inserted directly into the interlayer region of a layered double hydroxyl compound (LDH) to prepare an organic-inorganic hybrid antioxidant. AO-LDH has excellent antioxidant properties and has promising applications in the polymer field. LinaHuang et al. synthesized 3-(3,5-di-tert-butyl-4-hydroxyphenyl) propionic acid (DBHP) as an organic compound hindered phenolic antioxidant, which not only improved the dispersion stability of the prepared DBHP-ZnO nanoparticles in lubricants, but also scavenged the free radicals generated during the oxidation process of lubricants. The prepared DBHP-ZnO nanoparticles exhibited ideal dispersion, good thermal stability, and antioxidant properties in the base oil. The amino-functionalized silica gel was reacted with methyl acrylate and then repeatedly exchanged with ethylenediamine to synthesize 2.0-generation polyamide amine-modified silica gel (2.0G SG-PAMAM), and finally 2.0G SG-PAMAM was grafted with β-(3,5-di-tert-butyl-4-hydroxyphenyl) propionyl chloride to obtain new hindered phenolic antioxidants. phenolic antioxidants.
3.2 Phosphite antioxidants
Phosphite antioxidants can decompose hydroperoxides to produce stable substances to terminate the free radical chain reaction, and its non-volatile, low toxicity, low pollution, heat resistance and other advantages, more and more people pay attention to. Generally, phosphite antioxidants work better as auxiliary antioxidants in synergy with other main antioxidants. Mao Mengmei prepared three phosphite esters: tris(4-methyl-2-tert-butylphenyl) phosphite, tris(biphenyl) phosphite and tris(4-cinnamophenyl) phosphite. The antioxidant properties of the three phosphite antioxidants, tris(biphenyl) phosphite and tris(4-cinnamophenyl) phosphite, were verified in the processing of polypropylene.
Antioxidant 626 has good stability, maintain the stability of the process, product color stability, has a wide range of applications. Tetrakis (2,4-di-tert-butyl phenyl-4,4'-biphenyl) biphosphonate, known as antioxidant P- EPQ, is a large molecular weight antioxidant with good thermal stability, color stability and anti-hydrolysis properties. Phosphite antioxidant, the product is odorless, in the process of adding this type of antioxidant does not pollute the product, has good stability, and has good resistance to discoloration. However, most phosphate ester antioxidants thermal stability, hydrolytic stability is poor, the development of good performance, green process route phosphite antioxidant products are the main direction of development.
3.3 Amine antioxidants
Li Chaoming et al. prepared 3,6-di-tert-butylcarbazole by using chloro-tert-butane as an alkylating reagent and used it as an antioxidant for lubricants, which effectively improved the oxidative stability and thermal stability. Yao Junbing summarized several ways to improve the antioxidant capacity of amine antioxidants, such as oxidative coupling between amine antioxidants; synergistic with ashless thiocarbamate, zinc thiocarbamate, non-triphosphorous molybdate, and non-triphosphorous borate additives. Phenylenediamine antioxidants are mainly used in the field of fuel, and the process is complicated and mainly relies on imports. Zheng Jian has synthesized benzylamine antioxidants 180 and 182 with mild and efficient process conditions.
3.4 Compound antioxidants
By physically mixing and compounding different kinds of antioxidants, they have good synergistic antioxidant properties. Therefore, the synergistic antioxidant properties of different types of antioxidants are used to guide and design antioxidant molecules, and the main functional groups of different types of antioxidants, especially primary and secondary antioxidants, are combined into one molecule through chemical methods to improve antioxidant properties. Aromatic amine antioxidants and hindered phenolic antioxidants have good synergistic effects.
Zheng Jian synthesized a new phentolamine antioxidant (3,5-di-tert-butyl-4-hydroxyphenylmethyl) aniline with excellent thermal stability and antioxidant properties. Miao Changqing reacted di-tert-butyl peroxide with diphenylamine and 2,6-di-tert-butylphenol to prepare a synergistic amine and phenol antioxidant. Phenolic antioxidants can mainly capture oxidation radicals and inhibit oxidation reactions, while phosphite antioxidants can decompose hydroperoxides and thus play an antioxidant role. Therefore, the combination of two antioxidant functional groups, hindered phenol and phosphite, has the advantages of both hindered phenol space group effect and the function of decomposing hydroperoxides to achieve good synergistic effects.

4. Conclusion
The effect of hindered phenolic antioxidant is good. The machining process is stable, and the color change resistance is good, which is currently the most widely used, accounting for the highest proportion of the market antioxidant. However, due to its relatively small molecular weight, more volatilization during processing, and poor extraction resistance, its application has been limited. The effect of the amine antioxidant is good, but due to its high toxicity, environmental pollution, and poor color resistance, its application is limited. Phosphite antioxidant has significant heat resistance and resistance to discoloration, but the traditional phosphite antioxidant is easy to hydrolyze, limiting its storage and application. Therefore, it is necessary to improve the disadvantages of various antioxidants, synthesize the advantages, and improve the antioxidant capacity of antioxidants by means of compounding.
From: Chemical Engineering and Equipment, original author: Li Ruiduan, Pan Qian, Wang Jinli, etc.
Previous:
Next:
Copyright: Yingkou Fengguang Advanced Material Co., Ltd.Liao ICP Filing No. 17015501-1 Technical Support:![]() onnuoIAD
onnuoIAD
© COPRIGHT 2021 FENGGUANG ADVANED MATERAL.ALL RIGHTS RESERVED