
News
In 2020, it was rated as a national specialized and new "little giant" enterprise by the Ministry of industry and information technology
2023-10-11 484
Polyolefins are among the most widely used plastics globally, owing to their abundant raw materials, relatively low cost, and ease of processing, making them suitable for applications in packaging, automotive, construction, and consumer goods industries. Catalysts play a crucial role in the process of olefin polymerization. The right catalyst has a significant impact on improving the conversion rate of raw materials, reducing reaction time and process complexity, continuously enhancing the quality and performance of products, and expanding their application range. As the demand for polyolefins increases, research into olefin catalytic polymerization has become of paramount importance. The development of olefin polymerization catalysts can be categorized into four main types: Ziegler-Natta catalysts, metallocene catalysts, post-transition metal catalysts, and rare earth metal catalysts.
1. Ziegler-Natta Catalysts
In 1953, German scientist Karl Ziegler discovered that the TiCl4/AlEt3 system could catalyze the polymerization of ethylene at room temperature and atmospheric pressure, yielding low-density polyethylene with fewer branches. In 1954, Italian chemist Giulio Natta catalyzed the polymerization of propylene using the TiCl3/AlEt2Cl system, producing isotactic polypropylene for the first time. Their work marked a milestone in the history of catalytic chemistry, and the catalysts they developed are referred to as Ziegler-Natta catalysts.
催化剂组成 Catalyst Composition | 活性Kg pp/g cat Activity Kg pp/g cat | 等规度 % Isotacticity (%) | 产物形态 Product Form | 聚合工艺 Polymerization Process | |
第1代 1st Generation | TiCl3/AlEt2Cl | 0.8-1.2 | 88-91 | 不规则粉末 Irregular powder | 需后处理 Requires post-treatment |
第2代 2nd Generation | 处理的TiCl3/AlEt2Cl Modified TiCl3/AlEt2Cl | 3-5 | 94~97 | 颗粒 Granules | 需后处理 Requires post-treatment |
第3代 3rd Generation | TiCl4/MgCl2/苯甲酸酯/Al(C2H5)3 TiCl4/MgCl2/alkyl benzoate/Al(C2H5)3 | 15 | 90~95 | 规则颗粒,大小分布可控 Regular granules with controllable size distribution | 需脱无规物 Requires removal of irregular components |
第4代 4th Generation | TiCl4/MgCl2/邻苯二甲酸酯/Al(C2H5)3 TiCl4/MgCl2/ortho-phthalate/Al(C2H5)3 | 30 | 95~99 | 规则颗粒(球形),大小分布可控 Regular spherical granules with controllable size distribution | 不需后处理 No post-treatment required |
第5代 5th Generation | TiCl4/MgCl2/1,3-二醚/Al(C2H5)3 TiCl4/MgCl2/1,3-diglyme/Al(C2H5)3 | 60 | 95~99 | 规则颗粒(球形),大小可控 Regular spherical granules with controllable size distribution | 不需后处理 No post-treatment required |
Table 1 Performance of Ziegler-Natta Catalysts
The development of Ziegler-Natta catalysts can be divided into five generations, with Table 1 providing a performance comparison of catalysts from different generations. The first generation of Ziegler-Natta catalysts, as proposed by Ziegler and Natta, was industrialized by the Staffer company in 1959. Characteristics of first-generation catalysts include low catalytic activity, with the mass fraction of isotactic components like polypropylene only reaching 90%. The resulting polyethylene required chemical treatment to remove residual catalysts to meet usage requirements, involving complex processes such as deliming and removing amorphous components.
In the late 1960s, the second generation of Ziegler-Natta catalysts, referred to as "chelating catalysts" in China, emerged by introducing Lewis bases into the catalyst system. The distinguishing feature of the second-generation Ziegler-Natta catalysts was an increase in the catalyst's specific surface area, thereby enhancing catalytic activity and isotacticity. However, the catalytic activity remained relatively low, and the polymer still required processes such as deliming and removal of amorphous components. Additionally, the resulting polymers had relatively weak thermal oxidative stability and were not convenient for further processing.
In the late 1970s and early 1980s, the third generation of Ziegler-Natta catalysts was developed by loading titanium compounds onto activated magnesium chloride carriers. The third-generation Ziegler-Natta catalysts exhibited high catalytic activity, did not require deliming, and possessed high isotacticity.
In the mid-1980s, the fourth generation of Ziegler-Natta catalysts was introduced, utilizing ortho-phthalate as the inner electron donor and alkoxysilane as the outer electron donor in a spherical carrier system. These catalysts could control the physical and chemical properties of the carrier itself and the distribution of active centers on the carrier. This significantly improved catalytic efficiency, allowing the production of polyolefins with different molecular weights and molecular weight distributions. Consequently, they could produce polyolefin products with excellent morphology and high bulk density.
In the early 1990s, Himont introduced the first fifth-generation Ziegler-Natta catalysts, using 2,2-dialkyl-1,3-dialkoxypropane (1,3-dioxane) as the inner electron donor. This type of catalyst exhibited double the activity of ester-based catalysts and did not require external electron donors during polymerization. These catalysts are known as the fifth generation of Ziegler-Natta catalysts. Although dioxane-based catalysts demonstrated superior activity and hydrogen response compared to ester-based catalysts, they were costlier and less adaptable to existing polypropylene facilities. As time has progressed, new types of olefin catalysts have continued to emerge. However, Ziegler-Natta catalysts still maintain their importance in industrial applications.
2. Metallocene Catalysts
In the early 1980s, researchers Kaminsky and Sinn introduced methylalumoxane (MAO) as a co-catalyst along with metallocene compounds in homogeneous catalyst systems primarily based on zirconium. These catalysts were applied to ethylene polymerization and exhibited exceptionally high activity. This marked the beginning of the research boom in metallocene catalysts. Metallocene catalysts offer several advantages over traditional Ziegler-Natta catalysts. They possess higher catalytic activity, often surpassing traditional polypropylene catalysts by 1-2 orders of magnitude. Metallocene catalysts feature a single active center, resulting in polymers with narrow molecular weight distributions. They also exhibit excellent copolymerization capabilities, allowing for the co-polymerization of various olefins with ethylene to produce a wide range of new polyolefin materials. Additionally, copolymerized monomers are evenly distributed within the polymer's main chain. Apart from these features, metallocene catalysts have broader applicability, as they can catalyze not only common α-olefins but also cyclic olefins like norbornene.
3. Post-Transition Metal Catalysts
In 1995, Brookhart achieved high catalytic activity in ethylene polymerization using ligands derived from diamine, nickel, and palladium ions. Further research revealed that many coordination compounds formed by various organic groups and metal atoms without cyclopentadienyl ligands could also catalyze olefin polymerization with high reactivity.
Post-transition metal catalysts are relatively easy to synthesize, stable in performance, cost-effective, and yield stable and highly active complexes. The catalyst's performance can be significantly influenced by the ligand's spatial structure and electronic properties. Thus, catalyst activity and polymer properties can be controlled by adjusting the ligand structure. However, post-transition metals have some drawbacks, such as poor oxygen affinity.
4. Rare Earth Metal Catalysts
The development of rare earth coordination polymerization catalysts can be summarized in two aspects: single olefin polymerization and diolefin polymerization. Single olefin polymerization research began earlier and has made significant progress, with a clear understanding of the active center models. Diolefin polymerization started in 1964, and research in this area has been conducted mainly in China, the Soviet Union, Europe, the United States, and Japan. Chinese scholars have made notable progress in understanding the active center models. Rare earth elements possess characteristics such as partially filled 4f electron orbitals and lanthanide contraction, making them exhibit unique catalytic performance when used as catalyst components. Currently, research on rare earth metal catalysts, both theoretically and experimentally, is relatively limited, providing researchers with ample research space and new opportunities.
5. Outlook
Over several decades of development, polyolefin catalysts have evolved significantly. Some catalysts have been well-developed, such as Ziegler-Natta catalysts and metallocene catalysts. In the future, new active centers may be discovered, leading to the formation of novel polyolefin catalyst systems. New types of ligands are likely to emerge, expanding the variety of catalysts available. Alternative chain structures may be uncovered, resulting in new multi-nuclear polyolefin catalysts. Polyolefin catalysts are poised for rapid advancements in the years to come.
References:
1. Olefin Coordination Polymerization Catalysts and Polyolefins
2. Advances in Olefin Polymerization Catalysts
3. Progress in Polypropylene Catalyst Research
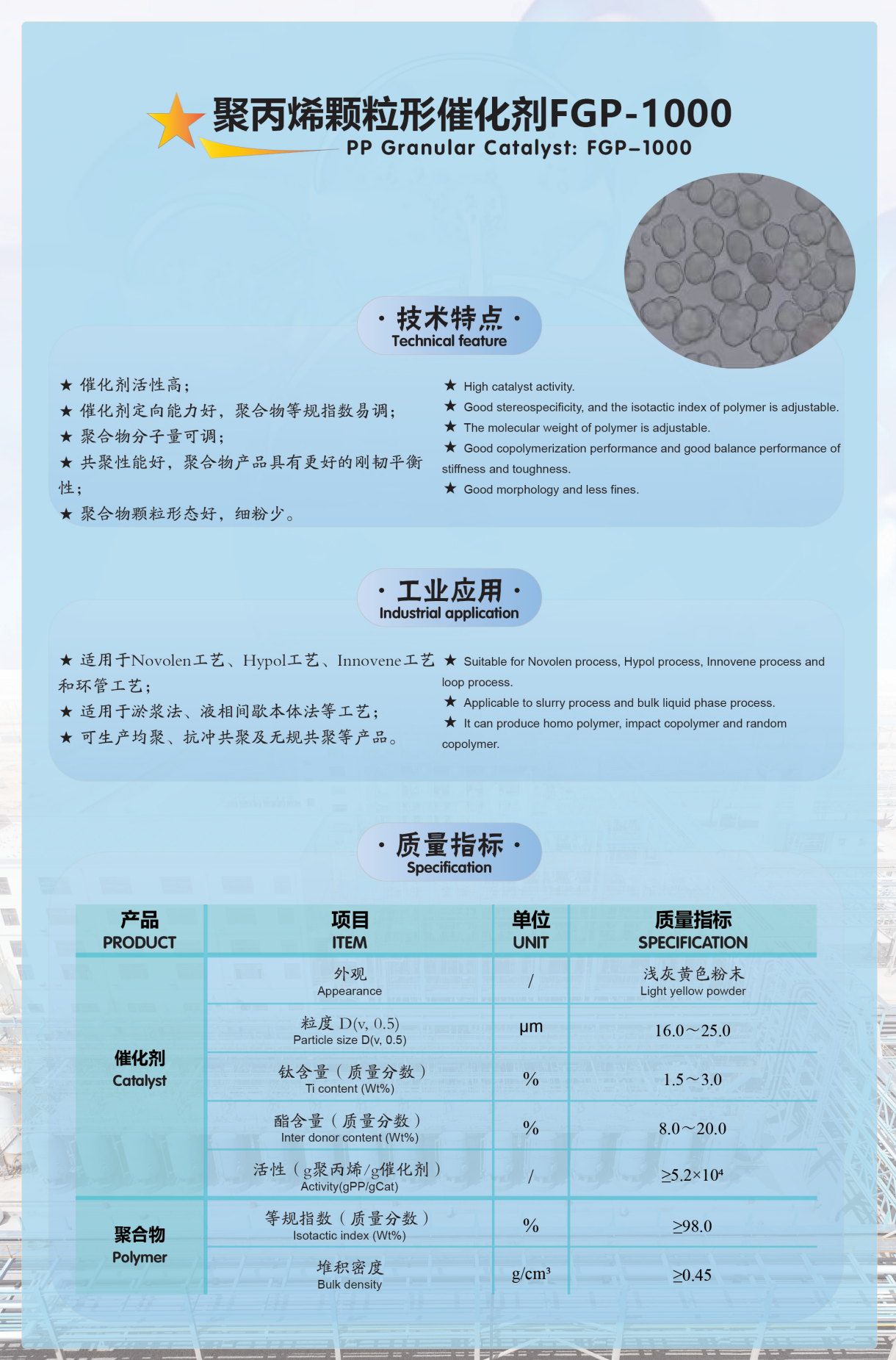
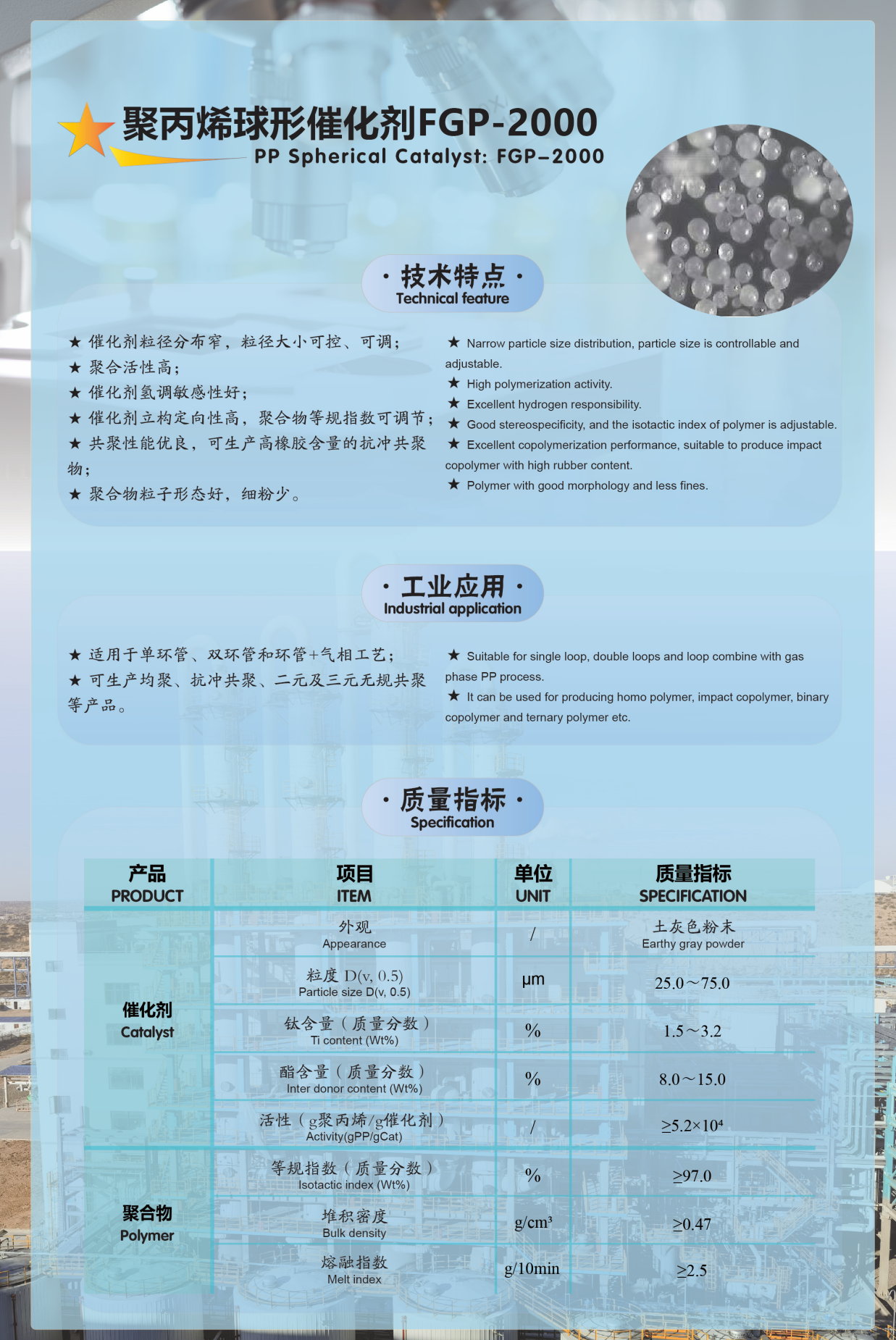
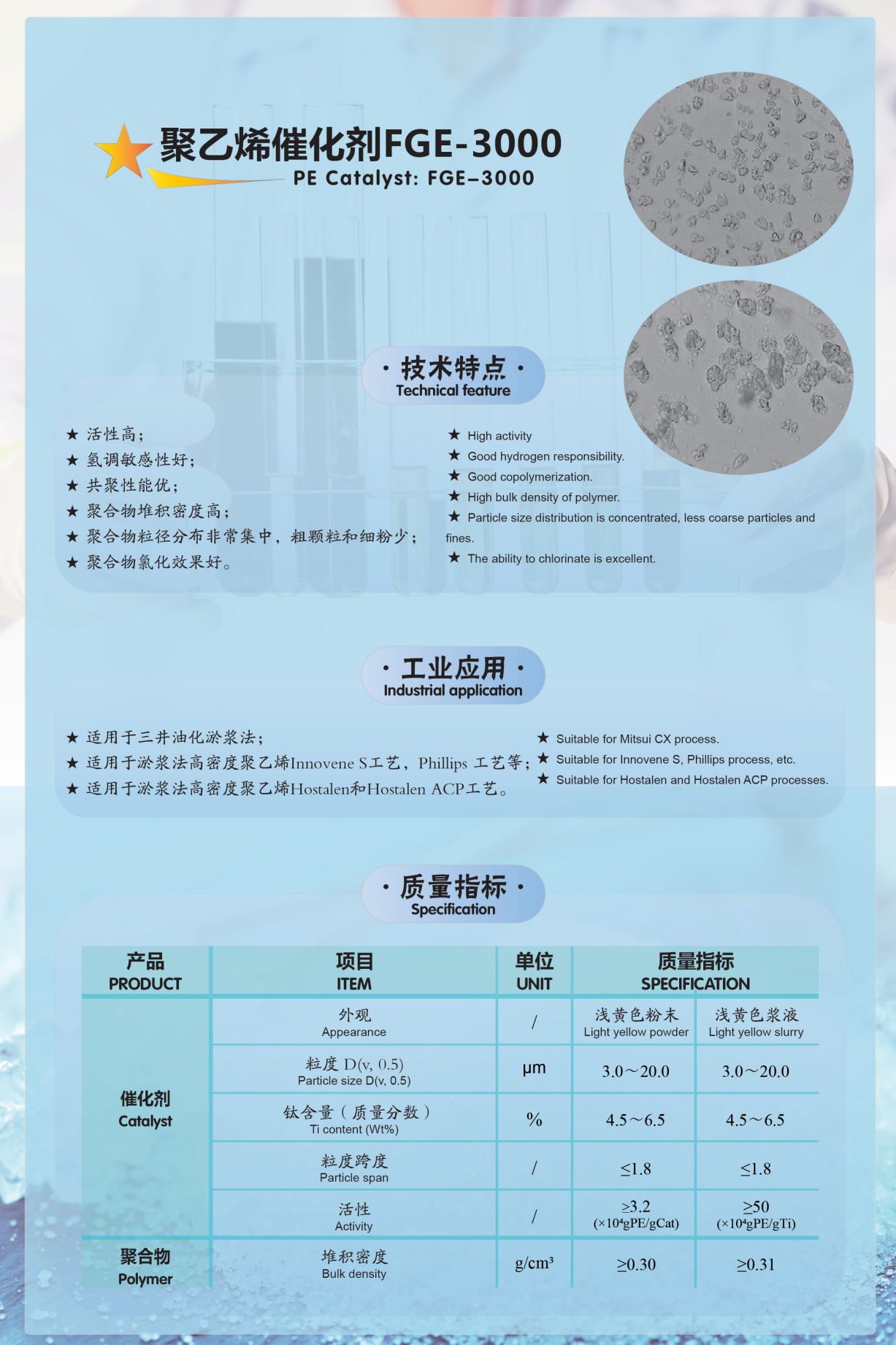
Appendix: Introduction to Our Company's Olefin Polymerization Catalyst Products
Previous:
Next:
Copyright: Yingkou Fengguang Advanced Material Co., Ltd.Liao ICP Filing No. 17015501-1 Technical Support:![]() onnuoIAD
onnuoIAD
© COPRIGHT 2021 FENGGUANG ADVANED MATERAL.ALL RIGHTS RESERVED